1. Phosphine Coordination Materials for Gas Storage, Separation, and Catalysis
Phosphine Coordination Materials are a new class of porous coordination polymers that is defined by the use of phosphine ligands to link between metal nodes. The phosphine site allows for a wide variety of both pre- and post-synthetic modifications, leading to the potential inclusion of catalytically active metals, guest-accessible polar groups (e.g. R3P=X, X = O, S, NR’, etc.), charged species (e.g. phosphonium alkyls, R3P+R’), and halogenated species (R3P-BX3, X = halogen). Such functionalization could lead to applications in numerous fields, including selective heterogeneous catalysis at single sites, gas sensing and separation, and small molecule sequestration.
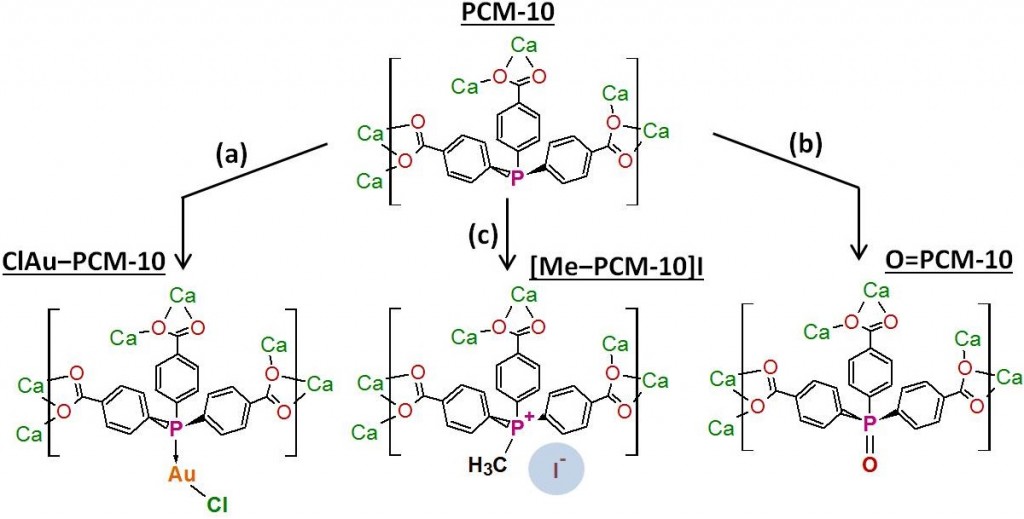
The free phospine site in PCM-10 can be post-synthetically modified by reaction with reagents such as (Me2S)AuCl (a), H2O2 (b), or CH3I (c) to add functionalities that affect the gas sorption properties of the material. The counteranion of the ionic Me-PCM-10 can be exchanged to further alter the properties of the PCM.
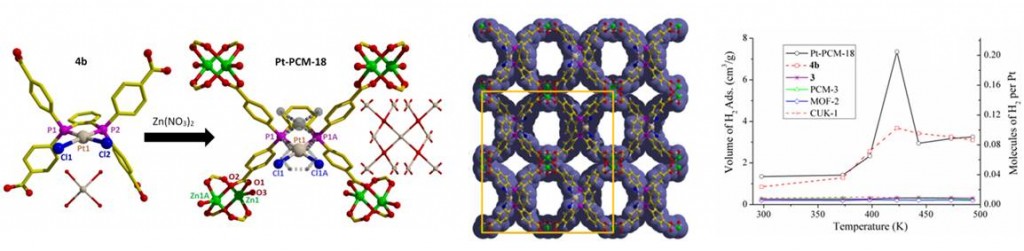
PCM-18, a material composed of bis(phosphine)–MCl2 complexes (M = Pd, Pt) and Zn2+ paddlewheel dimers (left); accessible pores of the PCM-18 lattice (center); unusual high-temperature H2 sorption of Pt-PCM-18 and PtCl2 complex (right)
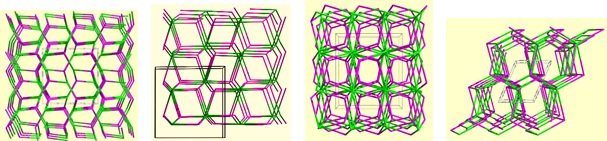
A nodal representation of a series of PCMs formed using different alkali hydroxides, but otherwise identical reagents and conditions. From left to right: PCM-6Li, PCM-7Na, PCM-8K/PCM-8Rb, and PCM-9Cs.
References:
- Tuning the Host-Guest Interactions in a Phosphine Coordination Polymer Through Different Types of Post-Synthetic Modification, A. J. Nuñez, Maxwell S. Chang, I. A. Ibarra, S. M. Humphrey, Inorg. Chem. 2013, 53, 282.
- Rational Design of Porous Coordination Polymers Based on Bis(phosphine)MCl2 Compelxes That Exhibit High-Temperature H2 Sorption and Chemical Reactivity, A. M. Bohnsack, I. A. Ibarra, V. I. Bakhmutov, V. M. Lynch, S. M. Humphrey, J. Am. Chem. Soc. 2013, 135, 16038-16041.
- Molecular Sensing and Discrimination by a Luminescent Terbium-Phosphine Oxide Coordination Material, I. A. Ibarra, T. W. Hesterberg, J. Chang, J. W. Yoon, B. J. Holliday, S. M. Humphrey, Chem. Commun. 2013, 49, 7156-7158.
2. Noble Metal Nanoparticles and Composite Catalyst Materials
The reproducible preparation of near-monodisperse samples of noble metal nanoparticles with defined surface structures, which can also be easily activated for applications in heterogeneous catalysis is an ongoing synthetic challenge. We are presently interested in using non-conventional routes such as microwave heating in combination with automated reaction apparatus to prepare single-metal, alloy, and core-shell nanocrystals of the group IX, X and XI metals. Noble metal alloys are currently of particular interest to the group, since alloys of conventionally immiscible metals like Rh and Au or Rh and Ag have shown to be significantly more catalytically active than rhodium by itself, despite gold and silver not being active themselves.
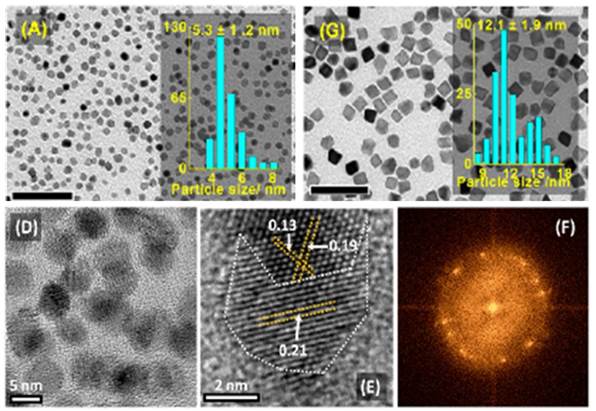
Fine tuning of several kinetic parameters allows for precise morphological control of Rh, Pd, and Pt nanoparticles (Rh pictured). Microwave heating also greatly improves the crystallinity of the particles.
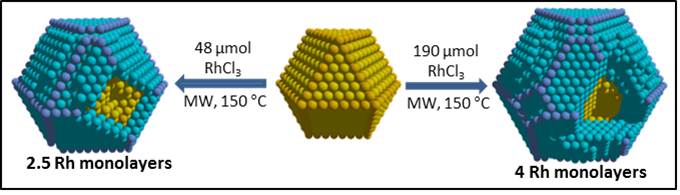
Innovative approach for the preparation of thermodynamically disfavored core-shell structures (Au@Rh and Ag@Rh) using microwave irradiation.
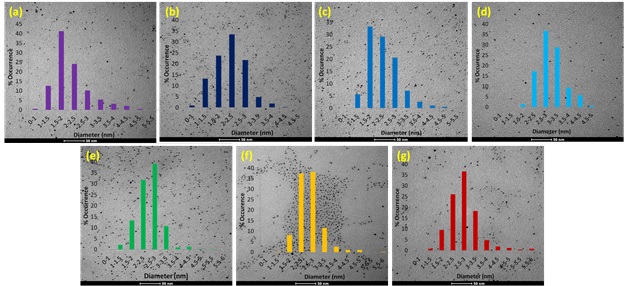
TEM images and size distributions for Pd50Au50 alloy nanoparticles synthesized using microwave heating after reaction times of 0.5, 2, 5, 15, 30, and 60 minutes, respectively.
We are also interested in the templated synthesis of mesoporous metal oxides, which have the potential to enhance the reactivity and selectivity of nanoparticles supported on them via electronic interactions with the nanoparticles and exclusion of potential reactants based on size. Some metal oxides are catalytically active in their own right, introducing the possibility of dual noble metal-metal oxide catalyst systems.
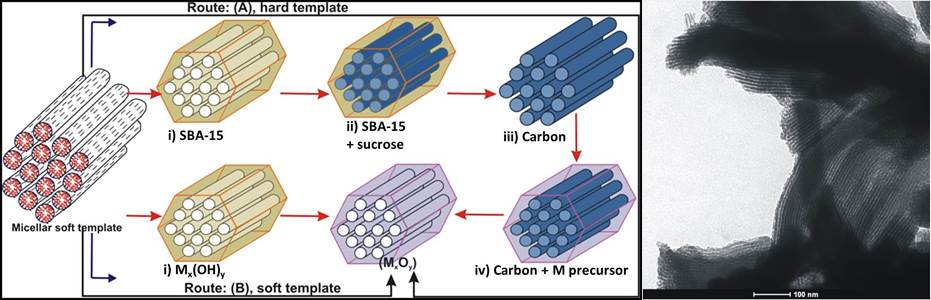
Mesoporous Co3O4: Synthesis scheme using both solid (“hard”) and micellar (“soft”) templates (left); TEM image showing ordered cylindrical channels (right).
References:
- Effect of microwave heating on the synthesis of rhodium nanoparticles in ionic liquids, S. Garcia, J. J. Buckley, R. L. Brutchey, S. M. Humphrey, Inorg. Chim. Acta 2014, in press.
- Microwave Synthesis of Au-Rh Core-Shell Nanparticles and Implications of the Shell Thickness in Hydrogenation Catalysis, S. García, N. Dahal, J. Zhou, H. Celio, A. Dolocan, S. M. Humphrey, Chem Commun. 2013, 49, 4241.
- Beneficial Effects of Microwave-Assisted Heating versus Conventional Heating in Noble Metal Nanoparticle Synthesis, N. Dahal, S. García, J. Zhou, S. M. Humphrey, ACS Nano. 2012.
- High Surface Area Mesoporous Co3O4 from a Direct Soft-Template Route, N. Dahal, I. A. Ibarra, S. M. Humphrey, J. Mater. Chem. 2012, 22, 12675.
